Table of Contents
Humid air
Air humidity - everyone can sense it…. but very often in an incomplete way at best, unfortunately. We will explain this here; here is also a short film on this.1)
Moisture/humidity is simply another word for water and is used when it is present in other substances mixed as water. We speak of air moisture (the amount of water vapour present in the air) or of material moisture (the water which is contained in microscopic empty spaces in a material called “pores”). Moisture - that is water - is present almost everywhere in our surroundings, usually in a beneficial proportion: neither too moist (which allows mould growth), nor too dry (which is not good for our mucous membranes). First it must be stated: the relative humidity in the air in indoor spaces that are occupied by humans should be within the range of 35 to 60% 2) on average. (It will be explained in this section what relative humidity actually is – many errors are based on a false understanding of relative humidity).
Let us start with water: for practical use, it is enough to know about the three states of matter of water. Solid in form like ice, liquid (as liquid water from the tap) and gas (water vapour).
Water consists of the molecular compound H2O.
In water vapour these molecules have so much space that they rarely collide with each other (and other molecules).
In liquid water the molecules are very close together, they are “held” together by electrical forces, but can still be easily pushed against each other.
In contrast, in solid ice the molecules are joined together in a fixed lattice. This molecular adjustment helps us understand more easily the processes (evaporation, melting) known to us from handling water in practice. For this we only need to introduce a model representation: the understanding of heat as a microscopically distributed “chaotic” energy, particularly in the form of kinetic energy of the molecules.
This understanding arose in physics towards the end of the 19th century and is also called the kinetic theory of heat 3). Today, every child learns this at some time in school – at that time this was a revolutionary explanation. It is a good example of the assessment: “There is nothing more practical than a good theory”.
The faster the vibration of the molecules, the more thermal energy they contain; the average kinetic energy of the molecules is the exact measure for the temperature – with which a quantifiable variable already known in mechanics can be used to explain the phenomena of “thermodynamics/study of heat” known from ancient times. The processes involved in the evaporation of a liquid and even some quantitative aspects of these can be illustrated based on the kinetic theory.
Let us observe the evaporation process in a container that is sealed all around (e.g. a corked bottle), which is e.g. filled 40 % with liquid water and is kept at a constant temperature (e.g. by means of thermostatically regulated heating). In the rest of the container there is air or some other gas for example, its density does not matter. The container has already been illustrated in the figures above.
The water molecules in the liquid water vibrate depending on the prevailing temperature – this is an irregular distribution of the vibrating molecules, some molecules are faster, others are slower. Now and then some of these molecules may be fast enough to leave the surface. They will then be present as single water molecules in the space above the liquid, that is, there is now water vapour here. We call this process “evaporation”. However, the reverse process now also occurs: water molecules from the gas volume near the surface sometimes hit this surface and return to the liquid again. This process is called “condensation” or “formation of condensation”.
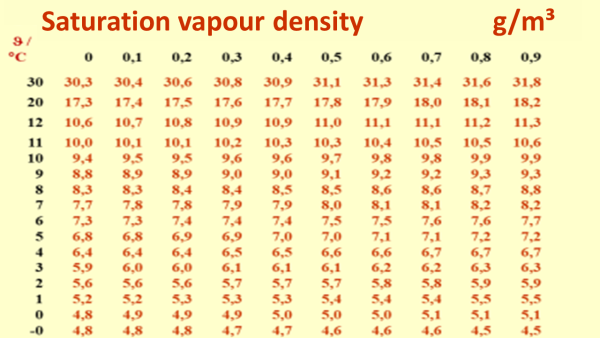
Of course, an equilibrium state is reached after a certain time (quite quickly!) A certain amount of water will then be in a gaseous state – it can't be more, because this will increasingly be compensated through condensation. This equilibrium water vapour concentration in the gas space above the water surface is called the “saturation vapour concentration”. At a temperature of 20 °C this is just 17.3 g/m³. That is a very small quantity of water – when at the same time it is clear that otherwise about 1,000 kg = 1,000,000 g of liquid water can be held by one m³. Water vapour at 20 °C thus holds about 58 thousand times the volume than liquid water of the same temperature - and that only if the volume is 100 % saturated with water vapour. Of course, if less water is available in total then there can always be a smaller quantity of water vapour than in case of saturation.
 | With a higher temperature thermal motion of the molecules becomes faster. For this reason the tendency towards the gas phase then increases: this means that the higher the temperature, the higher the saturation vapour density. |
 |
|
| Some values are given in this table for the saturation vapour density of water vapour. Hot air can hold much more water vapour than colder air. The saturation vapour density is the maximum density of water vapour which can exist in a given volume at that temperature4). If the saturation vapour density is exceeded, the water vapour begins to condensate on the condensation nuclei - small drops of liquid water are formed (called fog or cloud). This is commonly called “water vapour”, although this visible vapour is not water vapour at all, rather it is fog. The actual vaporous water (single molecules) is a completely transparent colourless gas. On the other hand, less water vapour than the saturation volume may well occur in a gas volume, for this e.g. one would only have to dilute a given air volume with dry air (i.e. air without any water molecules). | |
 |
|
| The vapour (pressure) curve - the course of the saturation vapour density over the temperature. Intermediate values are also possible with this - the value 17.3 g/m³ belonging to 20°C is indicated here. For understanding this, it is helpful if this is seen in relation to the liquid water quantity that is possible in one cubic meter. At 20°C this is about 1000 kg, i.e. about 58 thousand fold. Vaporous water has a much lower density than liquid water, and the colder it is, the fewer the vapour molecules which can fit into this volume without resulting in the formation of condensation. | |
 | Here is the definition of relative humidity: This is the percentage of the absolute humidity (water vapour density) relative to the saturation vapour density (at the prevailing temperature).In short: the current water vapour density in relation to the maximum water vapour density at the existing temperature. |
 | Now it is immediately clear why the relative humidity rises when a gas volume cools down,in certain circumstances up to 100%… more than this is not possible as the “excess water” must then condense.This happens either on a cold surface (condensation)or on dust particles in the air (fog).The temperature, at which this occurs for an air quantity with a given absolute humidity is called the dewpoint temperature. Many often speak of this simply as the “dewpoint”, that's a temperature, not a place.4) |
Our “Building Physics Course: Humidity” continues with an easy-to-understand explanation of moisture transport in exterior building assemblies (German only). Equipped with this basic understanding, practical application is considerably facilitated; it turns out that there are good well-proven methods for relieving the load on the building structure and increasing comfort and saving energy, even with properly implemented interior insulation solutions for moisture protection.
The interrelationships described here make it easy to understand many processes: why some of the processes we observe in nature, in buildings and in technical systems take place the way they do5). Understanding these helps to avoid problems with condensation - and grasp the real significance of the “dewpoint” (contrary to the myths). We will illustrate this here using a few examples, in no particular order.
Examples of processes relating to humid air
Note for experts and those on the way to becoming one: on the page exercises relating to humid air (German only) we have compiled some tasks with which you can calculate for yourself the processes which are only “explained” here.
 Condensation on a cold surface | Example 1: A cold bottle is taken out from the fridge into the warm air in the kitchen (20°, 50% relative humidity; this has an absolute humidity of 8.65 g/m³). After a short time, water droplets can be seen on the surface of the bottle: the air near it can only 'hold' 6.8 g/m³ of water vapour at a temperature of 5°C; the remaining water has to go somewhere, so it condenses here, which is why droplets form on the bottle. |
| This example also explains many other similar processes: condensation on the lawn in the early morning, especially in autumn: the ground already becomes quite cold due to heat radiation into the sky, and the lawn is colder than the dewpoint temperature of the air6). The same may be observed on the windscreen of a car; here the water may even freeze if the temperature then falls below 0°C (formation of frost). | |
|
Cloud formation (source: NASA) | Example 2: If an amount of air that is saturated with water vapour to a large extent ascends (e.g. near a mountain range) then from a certain height onwards the air will cool down below the dewpoint temperature: that's when clouds begin to form. |
 Condensation on a cold water pipe | Example 3: An example that is important for building services systems is condensation on cold water pipes; if these are not insulated and are routed through a basement, then drops of water can often be observed on the pipe in the summer. Many non-experts will then think that the pipe is leaking; however, this is condensation, and the water is coming from the air! For this reason, it is standard technical practice today to also cover cold water pipes with a certain minimum level of thermal insulation; of course this must be vapour impermeable as otherwise the water vapour will still be able to reach the surface of the pipe and the water will then also penetrate the insulation material as well (cold pipes should be insulated in a diffusion-tight manner! And this must be continuous, without any gaps.). |
| How do I ventilate the basement? Because humans cannot assess absolute humidity, it is advisable to use a measuring device which shows the absolute humidity (or the dewpoint temperature). | Example 4: the “damp basement”. The idea is quite common that on hot summer days the already damp basement can at last “be aired”. The houseowners are then surprised to find that that's when the water starts to flow down the walls (in streams!) Against the backdrop of the saturation vapour density curve, this is easy to understand: on hot humid days the hot (and humid!) outside air (for example 30°C at 75% relative humidity, therefore 22.5 g/m³ absolute humidity) cools down on cold surfaces in the basement which have a temperature of 20°C. However, the saturation moisture is only 17.3 g/m³ - it is no wonder then that condensation flows down the wall. Point to note: drying of the basement is possible only in conjunction with regulation of air transportation based on absolute humidity, called “dewpoint regulation”.[Schnieders /Kellertrocknung] |
 Mould is avoidable! That is why we avoid thermal bridges… | Example 5: Mould growth on walls. This not uncommon, particularly in existing buildings - often appearing in the first winter after new windows with perfectly airtight lip seals have been installed. Because the users usually do not compensate for the increased airtightness through more window ventilation (which is actually necessary approximately every four hours), the air humidity in the indoor spaces increases. On cold places of exterior walls (thermal bridges - which are most likely in window reveals and also at outer corners, especially if furniture is also placed next to these) the temperature now falls below the dewpoint temperature or at least a critical level of moisture in the wallpaper is reached. Note: airtight windows should only be used if adequate air exchange is ensured 7) - and this means at least a hygienic level of ventilation that ensures protection against moisture. And of course, thermal bridges should be minimised! |
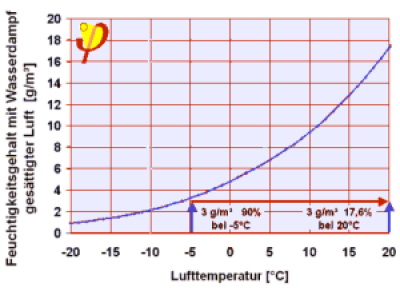 Air that has a relative humidity of 90% at a temperature of -5°C contains very little water vapour (2.9 g/m³)When heated to 20°C this is only 17% of the saturation vapour density; if such air is present in the room then it will only have a relative humidity of 17%. | Example 6: Ventilation in winter. In indoor spaces there are people present, who breathe out water vapour, among other things. In addition, there are wet towels, plants, cooking … all these produce humidity constantly. The indoor air therefore contains increasingly more water molecules per m³ than the outside air, i.e. the absolute humidity of indoor air is always higher than that of the outside air 8). If more fresh air from the outside is added to this 9) then it will become more dry inside the room. Completely incorrect views are prevalent regarding this: because the relative humidity of outside air in the winter is often “high” (90% is common!), many users think that they will be bringing “humid air” into the house. However, the outside air has only a low temperature and therefore the saturation vapour volume is very small (e.g. 5.9 g/m³ at 3°C). If this cold outside air is brought inside then it will heat up; at 20°C the saturation vapour density will then already be 17.3 g/m³, so that the relative humidity of the air that has just been brought inside and has become heated is now just 90% x 5.9 g/m³ / (17.3 g/m³) = 31%, which is quite dry! This incidentally is the main reason why indoor spaces are often “dry” in the main winter months; sometimes too dry, because less than around 35% causes stress for the respiratory tract. |
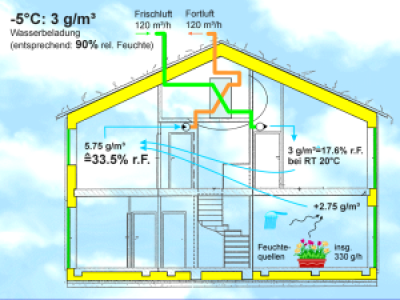 How to 'ventilate' properly: for 3 persons an air change rate between 80 and 120 m³ air per hour should be ensured. Moisture can be adequately removed in this way, and at the same time it will not become excessively dry - in a moderate winter climate. In locations with frequent severe frost (below -5°C for several days) additional humidification of the indoor air is advisable, or a moisture recovery heat exchanger can be used. | Example 7: Dehumidification through the exterior wall? On average one person gives off about 100 g water vapour in one hour; added to this is another equal amount due to activities such as cooking, washing and watering plants. This means about 5 kg (!) of water vapour in total per day. This water must eventually leave the building (otherwise it will become increasingly damp here). In Germany there are roughly about 50 m² of exterior wall area per person; if the building is traditionally constructed 10), then at the most about 5 g water vapour per square metre per day passes through the exterior wall through diffusion. This amounts to a total of 250 g per day, just 5% of the total amount that is given off. No matter how hard we try to achieve a build-up that is diffusion-open - the dehumidification contribution of the exterior walls is insignificant for the balancing of humidity in the building. A major part of the moisture introduced into the air must be removed via ventilation - and because we already have to ventilate sufficiently in any case, this is not a difficult task 11). However, the 5 g of water transported through 1 m² of wall each day can be a problem for the wall itself in the long run, if the emission of water vapour on the outside doesn't function sufficiently well. The outside layers of the wall should therefore be diffusion-open 12). |
It is quite interesting how much easier it is suddenly to comprehend everyday processes once the term “relative humidity” has been well understood. Of course, every energy consultant has to know all this and should be able to explain it. It would be helpful here to calculate for yourself a few examples based on the saturation humidity table (download here).
Literature
| [Schnieders 2009] | Schnieders, J.: Influence of basement insulation on moisture concentrations in basements, German only; Passive house institute; Darmstadt 2009. free Download (German only) from here |
Back to building physics basics
Go to solutions for moisture protection. (German only)





